Pilot Awards
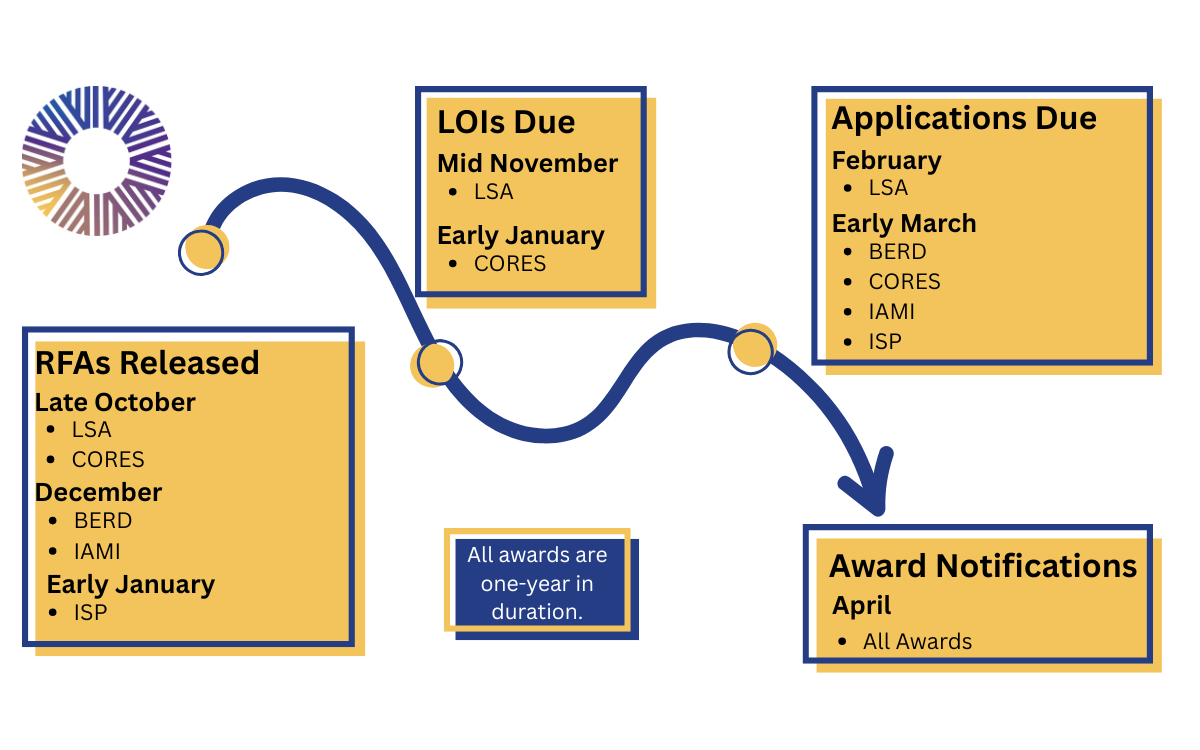
The Frontiers Pilot Program provides funding for early- and mid-career investigators to complete one (1) year-long, impactful, innovative, collaborative clinical and translational project that will support applications for subsequent externally funded larger studies. The Frontiers Pilot Program has two major goals: 1. to boost emerging investigators’ research programs to develop critical preliminary data in support of future applications, including studies leading to trials of promising treatments, interventions, devices, or novel diagnostic or analytic approaches; and 2. to support research that addresses clinical challenges pertinent to all populations and communities in the Frontiers catchment area of Kansas and Western Missouri and that in so-doing, can be applied to the general population.
Frontiers CTSI membership is required prior to submitting an application. Become a member and learn about member benefits.
NIH/NCATS Policy and Compliance
Additionally, Frontiers offers Biostatistics, Epidemiology and Research Design vouchers. Vouchers ($40,000 total per year, up to $5,000 per voucher) are open to any researcher from Frontiers partner institutions who have no access to other funding to support biostatistical assistance may apply for a voucher to pay for the cost of study design, data analysis, and bioinformatics. Contact the BERD navigator for details. The CTSI will follow up with each PI within 1 year after voucher approval with respect to publications, abstracts, or grant submissions related to the funded project.
Integrating Special Populations Pilot Award
Integrating Special Populations (ISP) Pilot Program provides grant funding and other support for clinical and translational research across a broad range of scientific disciplines. The objective of this pilot program is to support new and innovative ideas that will lead to externally funded awards. The Frontiers ISP Pilot Award addresses this goal by providing funds to help investigators carry out early-stage research addressing our primary aims 1. Improve health outcomes across the lifespan through multidisciplinary, multi-organizational teams that include patients, caregivers, clinicians, scientists, and other stakeholders and 2. Improve rare disease health outcomes by leveraging input from all stakeholders in study design.
One award is given annually for $25,000.
ISP Research Focus Areas
- Consequences of Maternal-Fetal-Child, and Adolescent disorders and disparities on the health of adults and older adults.
- Supporting Transitions of Care from childhood to young adult and adult to older adult.
- Incorporating participants who are older adults or rural residents in drug and other intervention development and testing.
- Addressing unique challenges of rare diseases and improving access to care and other resources for families in rural and underserved communities.
Application Materials
The Broderick Crawford Community Partnership Awards
The Broderick Crawford Community Partnership Award (BCCPA) supports the development of new academic-community partnerships or to strengthen existing partnerships. These awards will be used to facilitate community-based activities that build trust and strengthen relationships between researchers, patient advocates, community members and/or community-based organizations.
Applications are not currently being accepted.
Up to six awards issued annually; each award is up to $2,500.
Past awardees include:
- Caleb Stephens and Nicole Hodges
- Corien Shaw and Stacie Troshynski- Brown
- Kim Weaver, LeDarious Johnson and Jason Glenn
- Devin Smith and Meredith Scafe
- LaTanya Lipprand and Nuria Lara Castillo
- Brandon Clark and Emily Mailey
- Karynn Glover, Ciara Blakey, Jessica Welch and Roxie Montgomery
- Linda D'Silva and Marianna Ramirez
- Shalese Clay
- Matthew Kleinmann, Adrion Roberson, LaShone Releford, Angel Ferrara and Shaya Lockett
- Christy L'Esperance and Sharon Lindenbaum
- LaTanya Lipprand, Andrea Bradley-Ewing and Megan Krause, M.D.
- Christine Pacheco, Gaylene Crouser, Taneisha Scheuermann, Isaiah Brokenleg, Allen Greiner, Sarah Kessler, Byron Gajewski and Rachel DiTeresi
- John Tumberger and Stephani Stancil, Ph.D., APRN
- Dola Williams, Jill Peltzer, Ph.D., and Twoana Clark
- Tamara Falicov, Ph.D., Mang Sonna, Kay Heley, Alberto Villamandos, and Traci Angel
- Obie McNair, Ph.D., and Janette Berkley Patton, Ph.D.
- Karynn Glover, Ph.D., Roxie Montgomery, Jessica Welch, and Tania Jackson
- Amy Bodde, Ph.D., MPH, and Jessica Danon
- Jill Peltzer, Ph.D., RN, Dola Williams, Consuelo Ross, Kim Jones, Angela Williams, Hope Krebill, MSW, RN; Natabhona Mabachi and Crystal Lumpkins, Ph.D.
- Lisa Miche Lawson Ph.D., City of Fairway (Renee Reis), City of Merriam (Cole Surber) and City of Lenexa (Ryan Wiebe)
- Melissa McAtee, CPHE., Benjamin Grin, and Jaynell Assmann
- Nancy Rios, Jaime Perales Puchalt, Ph.D., Mariana Ramirez, LMSW
About Broderick A. Crawford:
Broderick Crawford was the President and Executive Director of the NBC Community Development Corporation. With over 30 years of experience in health care and community advocacy, Crawford was a national leader for community voice in research. He served on the Recruitment Innovation Center Community Advisory Board and was a Co-Principal Investigator on a large National Institutes of Health funded RADx-UP grant project in 10 Kansas counties. Many of us were fortunate enough to call him a friend and colleague and he more than deserves this award to be named in his honor.
Inter-Institutional Pilot Project Awards
The National Center for Advancing Translational Sciences’ (NCATS) Clinical & Translational Science Award (CTSA) program seeks to develop and implement innovative solutions that will improve the efficiency, quality, and impact of the process for turning observations in the laboratory, clinic, and community into interventions that improve the health of individuals and communities.
The CTSA program supports a national network of medical research institutions that work together to speed the translation of research discovery into improved patient care. Nine CTSA institutions have joined together to form the Consortium of Rural States (CORES): The University of Utah Health, the University of New Mexico Health Sciences Center, Frontiers Clinical and Translational Science Institute, the University of Kentucky, the Translational Research Institute at the University of Arkansas for Medical Sciences, the University of Iowa, Dartmouth University, Penn State University and South Carolina.
The purpose of this request for applications (RFA) is to promote multi-institutional collaboration across the CTSA consortium by funding innovative translational science research projects that involve two or more of these nine CTSA institutions. This pilot program is soliciting applications from faculty members at all career levels for translational science pilot projects that will exemplify the CTSA mission of “understanding a scientific or operational principle underlying a step of the translational process with the goal of developing generalizable principles to accelerate translational research.”
CORES Pilot Focus Areas
The CORES Inter‐Institutional Pilot Program will fund translational science projects aiming to identify and overcome challenges to the performance of translational research.
Pilots should articulate a clear translational research challenge and propose an innovative plan to overcome or ameliorate it (i.e., a translational science innovation). The proposed innovations should be broadly generalizable to many different translational research questions, and not specific to any one project or disease. Proposed projects should align with one of the following project scopes:
|
Develop:
|
New methodology, technology, tool, resource, or training paradigm that has generalizable application to an identified translational roadblock |
|
Demonstrate: |
New methodology, technology, tool, resource, or training paradigm to improve the effectiveness or efficiency of the translational process (including feasibility to support future clinical or translational science or research projects) |
|
Disseminate: |
Tools to effectively promote methodology, technology, tool, resource, or training paradigm that overcome an identified translational roadblock or improve the effectiveness or efficiency of the translational process into broader use |
Lauren S. Aaronson Frontiers Pilot Program
The Lauren S. Aaronson (LSA) Frontiers Clinical and Translational Research Pilot Program provides grant funding and other support to grow interdisciplinary, investigator-initiated clinical and translational research across a broad range of scientific disciplines. The objective of this pilot program is to support new and innovative ideas that will lead to externally funded awards.
Up to three awards are issued annually for up to $50,000 each.
LSA Research Priority Areas
Pilot projects are required to focus on translational science, i.e., understanding a scientific or operational principle underlying a step of the translational process with the goal of developing generalizable principles to accelerate translational research.
NCATS definitions:
- Translation: The process of turning observations in the laboratory, clinic and community into interventions that improve the health of individuals and communities – from diagnostics, preventions, and treatments to medical procedures and behavioral changes.
- Translational Research: The endeavor to traverse a particular step of the translational process for a particular target or disease.
- Translational Science: The field of investigation focused on understanding the scientific and operational principles underlying each step of the translational process.
Where translational research focuses on advancing a step of the translational process for a specific target/disease, translational science seeks to develop, demonstrate, and disseminate generalizable innovations and strategies to improve the process of translational research.
Translational science projects seek to 1) identify and understand barriers that delay progress or limit the quality, impact, or equity of translational research (e.g., clinical trial recruitment, data interoperability, implementation, etc.), and 2) develop innovative solutions (e.g., methods, best practices, tools, technologies) to overcome these barriers. Addressing critical barriers will allow subsequent translational research to accelerate the time from discovery to improved human health. The innovative solutions will have broad applicability to multiple research projects, increasing capacity and efficiency.
Applications directly related to one or more of the following areas will be accepted:
- Processes and programs to understand, support and advance translation, e.g., collaborative structures; workforce development; integration of project management; incentives/credit for team science; incentives/credit for health improvements; education/training (scientific and cultural); biomarker qualification process.
- Data-related improvements and tools, e.g., data interoperability; Electronic Health Records for research; data transparency/release.
- Clinical research improvements and tools, e.g., clinical trial networks; clinical outcome criteria (e.g., patient-reported outcomes); clinical diagnostic criteria; contemporary clinical trial designs; single Institutional Review Board (IRB) implementation; regulatory science; shortening time of intervention adoption.
- Clinical study recruitment improvements and tools, e.g., identification, recruitment, engagement and/or retention of populations and/or subpopulations in clinical trials and studies.
- Community and stakeholder engagement.
- Methods to better measure impact on health (or lack thereof).
The 2026 application process has closed.
Please make sure to review the National Institutes of Health's current priorities.
Trailblazer Awards
Frontiers offers Trailblazer Awards that provide funding to support targeted research.
Institute for Advancing Medical Innovation Trailblazer Awards
The Institute for Advancing Medical Innovation (IAMI) Trailblazer Award provides grant funding and other support for clinical and translational research across a broad range of scientific disciplines. The objective of this pilot program is to support new and innovative ideas that will lead to externally funded awards. The Frontiers IAMI Trailblazer Award addresses this goal by providing funds to help investigators carry out early-stage project development of novel drugs, devices, and diagnostics. The overall intent is to acquire research results that are disseminated in impactful publications, support successful extramural funding applications, and yield products to be entered into our product development pipeline.
Up to two awards are given annually for up to $25,000 each.
IAMI Research Focus Areas
Experimental Therapeutics Trials
Eligible research activities include a) clinical validation of a drug target; b) clinical pharmacology (e.g., drug metabolism, bioanalysis, pharmacokinetics, pharmacodynamics, pharmacogenomics, allometric scaling); c) clinical proof of concept of a novel therapeutic or a repurposed FDA-approved or abandoned drug. Eligible awardees must have demonstrated proof of principle in validated in vitro and/or in vivo preclinical models.
Drug and Medical Device Development
Eligible research activity includes high throughput screening; generation and optimization of small molecule lead candidates; discovery of macromolecule therapeutics; synthesis of sufficient quantities of active pharmaceutical ingredient required for animal testing; conduct of preclinical safety, pharmacokinetic and proof of principle studies in validated animal models of disease; formulation development (including analytical chemistry support); as well as creation of prototype medical devices as well as necessary device testing.
Biomarker Discovery and Validation
Eligible research activities include a) biospecimen collection; b) patient registry development; c) genetic, genomic, proteomic and metabolomic analysis of patient specimens, and d) analysis of patient specimens using established and investigational methods to discover and validate biomarkers of drug activity.
Entrepreneurship Activities Consistent with IAMI’s Mission
Eligible research activities defining the investment thesis for potential product development opportunities, including unmet medical need, proposed solution, market, competitive landscape, patent position and strategy, summary of existing data, product development and regulatory planning, marketing and licensing strategy, as well as support for obtaining key opinion leader input.
While not required, special consideration will be given to applications which address the CTSA priority of research into the needs of the rare disease community.
Biostatistics, Epidemiology and Research Design Trailblazer Award
The Biostatistics, Epidemiology, and Research Design (BERD) Trailblazer Award provides grant funding to support statistical, epidemiological, and data science investigations toward novel methods development that will improve clinical and translational research (CTR) inferences. BERD Trailblazer Awards support research projects for methods development for CTR.
One award is given annually for up to $20,000.
BERD Research Focus Areas
Frontiers BERD Trailblazer Awards support projects that address methodologies or analyses useful to the study of all diseases, and include academic disciplines such as biostatistics, epidemiology, bioinformatics, data science, and other quantitative areas that impact clinical and translational science.
Application Materials
Biostatistics, Epidemiology and Research Design (BERD) Vouchers
Vouchers ($40,000 total per year, up to $5,000 per voucher): Open to any researcher from Frontiers partner institutions to support biostatistical assistance may apply for a voucher to pay for the cost of study design, data analysis, and bioinformatics. Contact the BERD navigator for details. The CTSI will follow up with each PI within 1 year after voucher approval with respect to publications, abstracts, or grant submissions related to the funded project.
Learn about previous projects that BERD vouchers have supported!
Previously Funded Projects
Information on previously funded projects can be found on our news page.
 Funded Projects
Funded Projects
 Funded Projects
Funded Projects
 KL2 Scholar · Funded Projects
KL2 Scholar · Funded Projects
 KL2 Scholar · Funded Projects · News
KL2 Scholar · Funded Projects · News
 TL1 Trainee · Funded Projects · News
TL1 Trainee · Funded Projects · News
 KL2 Scholar · Funded Projects
KL2 Scholar · Funded Projects
 KL2 Scholar · Funded Projects
KL2 Scholar · Funded Projects
 Funded Projects · News
Funded Projects · News
 Funded Projects · News
Funded Projects · News
 Funded Projects · News
Funded Projects · News
 Funded Projects · News
Funded Projects · News
 Funded Projects · News
Funded Projects · News
 Funded Projects · News
Funded Projects · News
 Funded Projects · News
Funded Projects · News
 Funded Projects · News
Funded Projects · News
 Funded Projects · News
Funded Projects · News
 Funded Projects · News
Funded Projects · News
 News · In the Community · Funded Projects
News · In the Community · Funded Projects
 Funded Projects · News
Funded Projects · News
 Funded Projects · News
Funded Projects · News
 Funded Projects · News
Funded Projects · News
 Funded Projects · News
Funded Projects · News
 Funded Projects · News
Funded Projects · News
 Funded Projects · News
Funded Projects · News
 Funded Projects · News
Funded Projects · News
 Funded Projects · News
Funded Projects · News
 Funded Projects · News
Funded Projects · News
 Funded Projects · News
Funded Projects · News
 Funded Projects · News
Funded Projects · News
 Funded Projects · News
Funded Projects · News
 Funded Projects · News
Funded Projects · News
 Funded Projects · News
Funded Projects · News
 News · In the Community · Funded Projects
News · In the Community · Funded Projects
 Funded Projects · News
Funded Projects · News
 Funded Projects · News
Funded Projects · News
 Funded Projects · In the Community
Funded Projects · In the Community
 Funded Projects · News
Funded Projects · News
 Funded Projects · News
Funded Projects · News
 Funded Projects · News
Funded Projects · News
 Funded Projects · News
Funded Projects · News
 Funded Projects · News
Funded Projects · News
 Funded Projects · News
Funded Projects · News
 Funded Projects · News
Funded Projects · News
Eligibility
Funding opportunities are open to principal investigators who are eligible to receive federal funding and employed by any Frontiers partner institution:
- Children's Mercy Kansas City
- Kansas City University (all campuses)
- Kansas State University (all campuses)
- Saint Luke's Health System
- University of Kansas
- University of Kansas Medical Center (all campuses)
- The University of Kansas Health System
- University of Missouri-Kansas City
Academic applicants are encouraged to collaborate with community organizations to develop an application.
Early to mid-career investigators are encouraged to apply. Established principal investigators may apply but should strongly justify how the pilot project is a new line of inquiry.
Principal Investigators are allowed to apply for more than one funding mechanism but will only be allowed to accept one award annually. Applicants may serve as PI on only one application but may be included as collaborators on any number of applications.
Volunteer faculty, residents, fellows, or graduate students are generally not eligible to serve as principal investigators. United States citizens, non-citizen nationals, or those who have legal admission as a permanent resident are eligible. However, any questions regarding eligibility should be addressed to individual institutional grants management offices before submission.
Citing Frontiers
All publications and presentations need to acknowledge NIH grant support arising from any research project that used Frontiers resources. Please use the correct citation language below depending on when you received support and on the type of support received.
The University of Kansas received its initial Clinical and Translational Science Award (CTSA) from the National Institutes of Health (NIH) which provided funding from July 1, 2011 through June 30, 2016. The CTSA program transferred from the National Center of Research Resources (NCRR) to the National Center for Advancing Translational Sciences (NCATS) in December 2011. The University of Kansas received its second CTSA in September 2017 from NCATS with the award period ending July 2022. The University of Kansas received its renewed CTSA in July 2022 from NCATS with the award period ending June 2027.
Support received between 2022 - 2027:
Support (Full or partial support from the CTSA)
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under the Award Number UL1TR002366. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Mentored Career Development Awards (KL2)
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under the Award Number KL2TR002367. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Pre- and Post-doctoral Trainee Awards (TL1)
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under the Award Number TL1TR002368. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Support received between 2017-2022:
Support (Full or partial support from the CTSA)
This work was supported by a CTSA grant from NCATS awarded to the University of Kansas for Frontiers: University of Kansas Clinical and Translational Science Institute (# UL1TR002366) The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or NCATS.
Mentored Career Development Awards (KL2)
This work was supported by a CTSA grant from NCATS awarded to the University of Kansas for Frontiers: University of Kansas Clinical and Translational Science Institute (# KL2TR002367). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or NCATS.
Pre- and Post-doctoral Trainee Awards (TL1)
This work was supported by a CTSA grant from NCATS awarded to the University of Kansas for Frontiers: University of Kansas Clinical and Translational Science Institute (# TL1TR002368). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or NCATS.
Support received between 2011-2016:
Support (Full or partial support from the CTSA)
This work was supported by a CTSA grant from NCATS awarded to the University of Kansas Medical Center for Frontiers: The Heartland Institute for Clinical and Translational Research (# UL1TR000001). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or NCATS.
Mentored Career Development Awards (KL2)
This work was supported by a CTSA grant from NCATS awarded to the University of Kansas Medical Center for Frontiers: The Heartland Institute for Clinical and Translational Research (# KL2TR000119). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or NCATS.
Pre- and Post-doctoral Trainee Award (TL1)
This work was supported by a CTSA grant from NCATS awarded to the University of Kansas Medical Center for Frontiers: The Heartland Institute for Clinical and Translational Research (# TL1TR000120). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or NCATS.

